SBG003 (tobramycin and vancomycin extended-release solution) has promise to be a first-in-class local antimicrobial for prevention of surgical site infection (SSI) after high-risk procedures including colorectal, cardiac, and orthopaedic procedures. SBG003 is instilled in a surgical site after the procedure is completed, conforming to surgical site surfaces for direct delivery to potentially contaminated areas, including those which may be poorly accessible to antimicrobials dosed systemically. In combination, the antimicrobials released from SBG003 are effective against bacteria responsible for over 90% of SSIs. SBG003 has been found to be effective in preclinical models of colorectal and orthopaedic SSI, outperforming active controls. SBG003 has received Qualified Infectious Disease Product (QIDP) designation from the FDA for prevention of SSI in abdominal surgery, including colorectal surgery.
- SBG003 has potential for:
- Reducing infection risk following up to five million procedures in the US annually
- Enabling improvement in health economic outcomes in acute care settings
- Product advantages:
- Over 90% coverage of SSI-causing pathogens
- Capability for both superficial and deep application, critical for preventing the most serious SSIs
- Provides highly effective delivery at >100x MIC for 24 hr+
- Water-based – no solvents or chemical reaction
- Dissolves fully after drug release, with minimal acid generation or low molecular weight byproducts
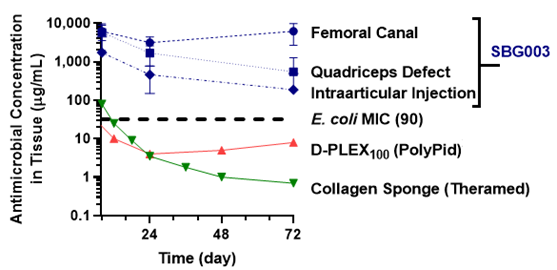
SBG003 Provides Superior Antibiotic Delivery
SBG003 data (Overstreet DJ et al, Drug Deliv Transl Res 2019): Total antimicrobial concentration in tissues adjacent to three model surgical sites in rabbits (n = 4). D-PLEX100 data are reported from rabbit abdominal wound fluid. (PolyPid website data) Collagen sponge data are reported from in canine synovial fluid. (Hayes GM et al, Vet Surg 2014)
SBG003 Is Effective in a Deep Abdominal SSI Model
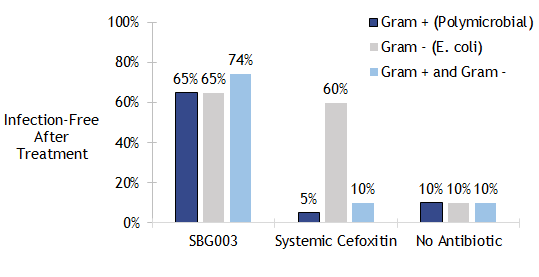
Percentage of infection-free rats six days after intraperitoneal inoculation with diluted bowel contents, E. coli, or both (n=20). SBG003 was effective in preventing infection caused by all three inoculum types.
Status and Development Plans
SBG003 is currently being evaluated in IND-enabling toxicology studies. In initial clinical studies, we plan to evaluate the safety and effectiveness of SBG003 in patients undergoing colorectal surgery. We are currently seeking to raise capital to support the development of SBG003 through Phase 2. For additional information on SBG003 and our development plans, please contact us.