Acute Postoperative Pain
Over 14 million patients undergoing surgical procedures annually in the United States require treatment for moderate to severe postoperative pain lasting at least 3 days. Aside from the impact of pain on a patient’s comfort and recovery, pain management frequently relies on opioids, which are associated with a host of serious risks to patients. Perhaps the most devastating risk is that perioperative opioid use can be an on-ramp for patients to begin new and persistent opioid use, which is reported to occur in 5-6% of adults after inpatient surgery and about 2% after outpatient surgery.
The primary shortcoming of currently available options for treatment of pain is inadequate efficacy beyond 24 hr, leaving a window of time when moderate to severe pain emerges and additional medication is needed.
SBG004: A Potential Best-in-Class Local Anesthetic
Achieving 72 hr analgesia with a local anesthetic requires sustaining effective local anesthetic concentrations in a volume of tissue surrounding the surgical site. SBG004 (bupivacaine in SB Gel) is uniquely suited to accomplish this. The temperature-responsive property of SB Gel enables it to be injected similarly to plain local anesthetic solution but then form a soft solid depot that is retained at the injection site, ensuring drug release that occurs throughout local tissue over its entire time course.
Competing approaches have one of two key drawbacks for local anesthetic release: either they rely on diffusion from the wound space resulting in limited drug distribution in tissue, or they are prone to dilution resulting in off-target release.
- SBG004 has potential for:
- Covering the full duration of moderate-to-severe pain
- Enabling reduction in opioid prescription and use
- Enabling safer and more reliable recovery and discharge to self-care
- Formulation advantages
- Ready-to-use presentation
- Effective in treating pain for 72+ hr, superior to plain local anesthetics and competing specialty products.
- Longer duration and more thoroughly distributed local anesthetic release than competing products.
- Pharmacokinetics indicative of extended release over 7+ days with minimal initial burst.
SBG004 Provides Analgesia For Up to Five Days
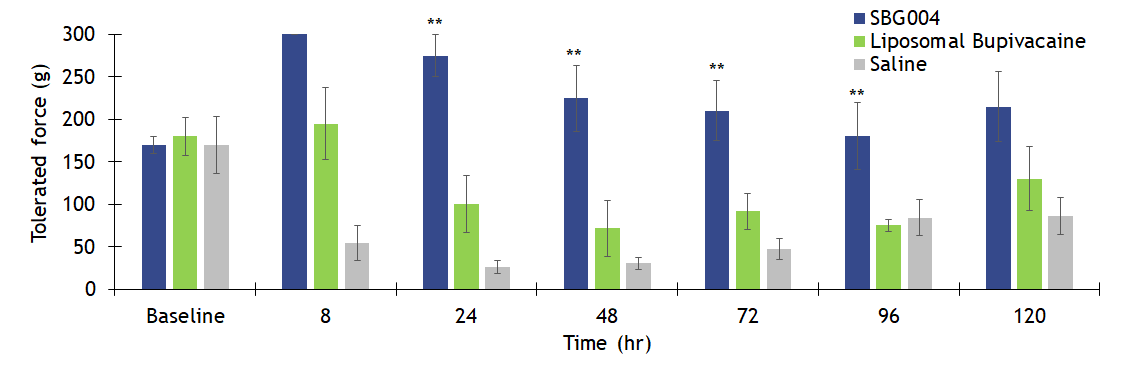
Mean force tolerated 0.5 cm from the incision following skin and muscle incision in minipigs (n=8, mean ± SEM). All treatments were dosed by subcutaneous injection. Methods were adapted from Castel et al. Eur J Pain, 2014. Double asterisk (**) denotes p < 0.05 vs. saline and liposomal bupivacaine.
Publications:
- Heffernan JM, McLaren AC, Glass CM, Overstreet DJ. Extended release of bupivacaine from temperature-responsive hydrogels provides multi-day analgesia for postoperative pain. Pain Medicine. 24(2): 113-121 (2023). [link]
- Overstreet DJ, Zdrale G, McLaren AC. Extended Release of Bupivacaine from Temperature-Responsive PNDJ Hydrogels Improves Postoperative Weight-Bearing in Rabbits Following Knee Surgery. Pharmaceuticals 17(7): 879 (2024). [link]
Status and Development Plans
SBG004 is currently in preclinical development. In clinical studies, we plan to evaluate SBG004 in model hard and soft tissue procedures similar to those used to support approval of other extended-release local anesthetics. We are currently seeking to raise capital to support the development of SBG004 through Phase 2. For additional information on SBG004 and our development plans, please contact us.
